In recent years, medications containing semaglutide—such as Ozempic and Wegovy—have surged in popularity as treatments for type 2 diabetes and obesity. But as their popularity and use increased, so did reports concerning gastrointestinal side effects, including gastroparesis, intestinal blockages, and ileus, leading many people to file Ozempic lawsuits.
Now, a new study from Harvard University researchers has raised concerns about another potential adverse event with these drugs—a rare but serious vision disorder.
Understanding the Study's Findings
The study, published in JAMA Ophthalmology, suggests that individuals taking semaglutide may be at an increased risk of developing nonarteritic anterior ischemic optic neuropathy (NAION). This condition can lead to sudden vision loss. Here are the key points from the research:
- Patients taking semaglutide were found to be 4 to 8 times more likely to develop NAION compared to those using other medications for diabetes and weight loss.
- Among patients with type 2 diabetes, the 36-month incidence of NAION was 8.9% for semaglutide users, compared to 1.8% for non-users.
- For individuals who were overweight or obese, the rates were 6.7% for semaglutide users and 0.8% for those on other treatments.
While these findings are concerning, they are preliminary and require further investigation. The researchers themselves have called for larger, more comprehensive studies to confirm their results.
If you are taking Ozempic or Weygovy and experience vision changes, reach out to our legal team to discuss your potential legal rights.
What is NAION?
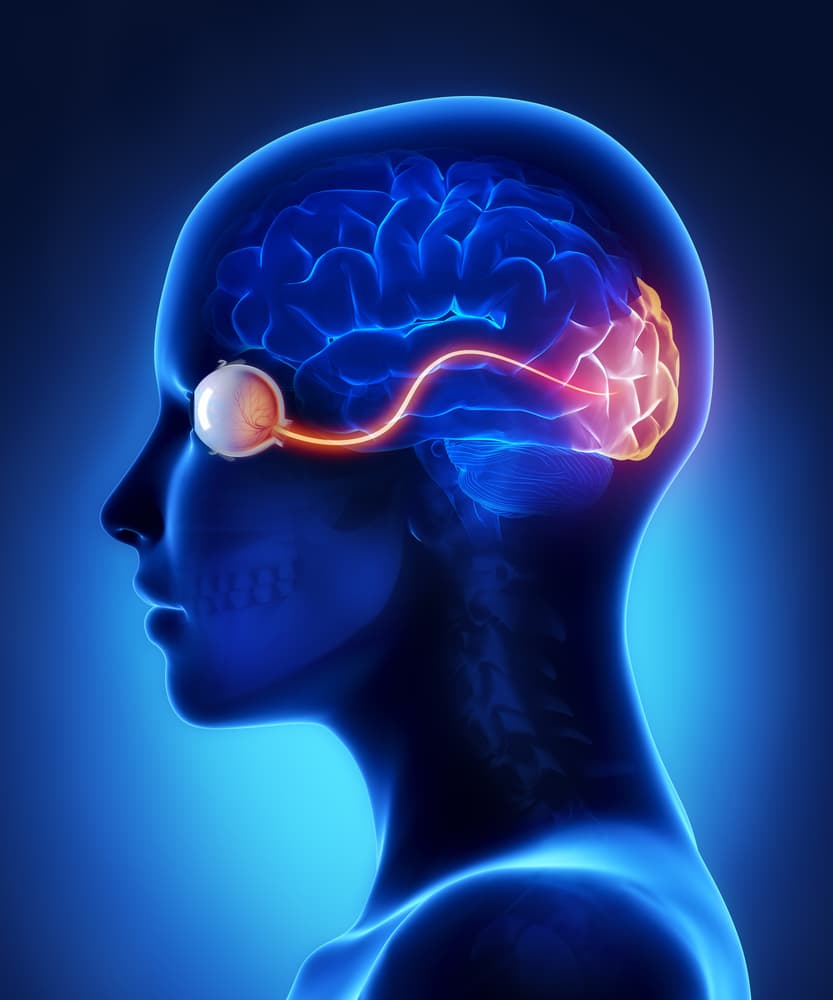
Nonarteritic anterior ischemic optic neuropathy (NAION) is a rare but serious condition affecting the optic nerve. Here's what patients should know:
- NAION is the most common acute optic neuropathy in individuals over 50 years old.
- It causes sudden, often irreversible vision loss in one eye, typically noticed upon waking.
- The condition results from a lack of blood flow to the optic nerve.
- NAION affects approximately 2-10 per 100,000 people.
- It is the second most common cause of blindness due to optic nerve damage after glaucoma.
While the exact causes of NAION are not fully understood, it has been associated with various risk factors, including diabetes, hypertension, and high cholesterol—conditions that many patients taking semaglutide may already have.
NAION Symptoms and Prognosis
Nonarteritic anterior ischemic optic neuropathy is characterized by sudden, painless vision loss in one eye. The primary symptoms include:
- Sudden vision loss: Typically occurs upon waking, affecting one eye.
- Visual field defects: Often manifest as loss of vision in the lower or upper half of the visual field.
- Reduced visual acuity: Ranging from mild to severe impairment.
- Impaired color vision: Particularly in the affected eye.
- Optic disc edema: Swelling of the optic disc, visible during an eye examination.
- Absence of pain: Unlike some other ocular conditions, NAION is typically painless.
- Relative afferent pupillary defect (RAPD): The affected eye shows a reduced pupillary response to light.
Symptoms are usually unilateral (affecting one eye), but in rare cases, both eyes can be affected simultaneously or sequentially. About 40% of patients may show some visual improvement within the first six months, while around 20% experience further deterioration. When improvement does occur, it’s usually modest. Significant recovery of vision is uncommon. About 15-20% of patients with NAION in one eye risk NAION occurring in their other eye over five years.
Treatment for NAION typically aims to prevent further episodes and manage underlying risk factors. Currently, there is no proven treatment to reverse the vision loss from NAION. Early diagnosis and management of risk factors are crucial in mitigating long-term impact and preventing the involvement of the other eye.
Semaglutide and GLP-1 Receptor Analogues: A Closer Look
Semaglutide belongs to a class of medications known as glucagon-like peptide-1 (GLP-1) receptor analogs. These drugs have gained popularity due to their effectiveness in managing diabetes and promoting weight loss. Some key points about these medications include:
- Semaglutide is the active ingredient in both Ozempic (used for diabetes and off-label for weight loss) and Wegovy (used for weight loss).
- Other drugs in this class include tirzepatide (brand name Mounjaro).
- These medications have shown significant efficacy in controlling blood sugar levels and aiding weight loss.
- Due to their effectiveness, prescriptions for these drugs have increased dramatically in recent years.
Implications for Patients
If you are currently taking Ozempic, Wegovy, or another semaglutide-containing medication, it's crucial not to panic or discontinue your medication without medical advice. Consider the following steps:
- Consult Your Healthcare Provider: Discuss the potential risks and benefits of continuing your medication regimen.
- Be Aware of Symptoms: Familiarize yourself with the signs of NAION, which include sudden vision loss, typically in one eye.
- Regular Eye Exams: Consider scheduling more frequent eye examinations to monitor for any changes in your vision.
- Report Any Changes: If you experience any sudden changes in your vision, seek immediate medical attention.
The Importance of Further Research
While this study raises important questions, more research is needed to establish a definitive link between semaglutide and NAION. The study authors themselves have called for:
- Larger retrospective, multicenter population-based studies
- Prospectively designed randomized clinical trials
- Postmarket analysis of all GLP-1 receptor agonists
One challenge in conducting these studies is the lack of a specific diagnostic code for NAION, which may complicate large-scale data collection. In the Harvard study, researchers had to manually review records, finding that 40% of cases initially marked as ischemic optic neuropathy were not actually NAION.
Current Labeling and Regulatory Status
As of now, NAION is not listed as a side effect on the labels of Ozempic or Wegovy. However, if further studies confirm the link between semaglutide and increased NAION risk, we may see changes in labeling requirements and potentially in prescribing practices.
The FDA and other regulatory bodies worldwide will likely be monitoring this situation closely. Any future regulatory actions could have significant implications for patients, healthcare providers, and pharmaceutical companies.
Balancing Benefits and Risks
It's important to remember that medications like Ozempic and Wegovy have provided significant benefits to many patients struggling with diabetes and obesity. These conditions themselves carry serious health risks, including cardiovascular disease, kidney problems, and other complications.
The potential risk of NAION must be weighed against the known benefits of these medications and the risks of untreated diabetes or obesity. This underscores the importance of personalized medical care and the need for patients to have open, informed discussions with their healthcare providers.
Equally important is the responsibility of pharmaceutical manufacturers to prioritize patient safety. If a link between these drugs and NAION is established, it is crucial that the manufacturers promptly and clearly warn both consumers and healthcare professionals about this potential risk. This information is vital for making informed decisions about treatment options and for implementing appropriate monitoring protocols.
The duty to warn is a fundamental principle in pharmaceutical liability law. Manufacturers have an ongoing obligation to update their product labeling and communicate new safety information as it becomes available. Failure to do so could potentially be seen as negligence and could form the basis for legal action if patients suffer harm as a result.
Looking Ahead: What's Next?
As we await further research and potential regulatory responses, several key developments may unfold:
- Additional Studies: We can expect to see more research focused on the potential link between semaglutide and NAION, as well as investigations into whether this risk extends to other GLP-1 receptor analogs.
- Regulatory Review: The FDA and other global health authorities may initiate reviews of the safety data for semaglutide and related medications.
- Updated Guidelines: Professional medical associations may issue new or updated guidelines for monitoring patients on these medications.
- Legal Developments: If a causal link is established, we may see legal actions on behalf of affected patients.
Lawsuits Targeting GLP-1 Agonists
While the potential link between semaglutide and NAION is a recent development, GLP-1 agonists are already the subject of numerous lawsuits due to other serious side effects. These legal actions involve not only Ozempic and Wegovy but also other drugs in this class, such as Mounjaro, Zepbound, Trulicity, Saxenda, Rybelsus, and Victoza.
The primary focus of current lawsuits against manufacturers of GLP-1 agonists is severe gastrointestinal side effects, including:

- Gastroparesis: Often referred to as stomach paralysis, this condition causes delayed stomach emptying and can lead to:
- Severe nausea and vomiting
- Abdominal pain and bloating
- Loss of appetite and weight loss
- Malnutrition and dehydration
- Difficulty eating and absorbing nutrients
- Ileus: Similar to gastroparesis but affecting the small intestine, ileus occurs when the intestinal muscles fail to move food properly through the digestive tract.
- Intestinal pseudo-obstruction: This condition mimics an intestinal blockage but is actually caused by improper functioning of the intestinal muscles.
FDA Action and Ozempic Warning Label Concerns
In September 2023, the FDA required Novo Nordisk to include information about ileus in Ozempic's warning label. However, as of August 2024, these severe gastrointestinal risks were not mentioned in the label highlights on the first page of the product information.
Multidistrict Litigation (MDL) Consolidates Federal Ozempic Lawsuits
On February 2, 2024, the Judicial Panel on Multidistrict Litigation ordered the consolidation of all Ozempic and other GLP-1 medication cases in the United States District Court for the Eastern District of Pennsylvania. This consolidation, known as Multidistrict Litigation, offers several benefits, including:
- Reduced costs and expenses for plaintiffs
- More efficient pursuit of claims
- Uniform pre-trial decisions from a single judge
Do I Have Grounds for Legal Action?
If you or a loved one has been diagnosed with any of the following conditions after taking Ozempic, Wegovy, Mounjaro, Zepbound, Trulicity, Saxenda, Rybelsus, or Victoza, you may be eligible to file a legal claim against the drug manufacturers, Novo Nordisk and Eli Lilly:

- Gastroparesis
- Intestinal blockage
- Bowel obstruction
- Ileus
- Intestinal pseudo-blockage
As the landscape of GLP-1 agonist litigation evolves, including potential future claims related to NAION, Cory Watson Attorneys remains committed to advocating for patient safety and holding pharmaceutical companies accountable for the harm their products may cause.
To Learn More About Bringing a Possible Ozempic Lawsuit, Contact Cory Watson Attorneys
The potential link between semaglutide and NAION is a developing story that underscores the complex nature of pharmaceutical safety. While these medications have proven benefits, this study highlights the importance of ongoing research and vigilance in monitoring for potential dangerous side effects.
At Cory Watson Attorneys, we remain committed to advocating for patient safety and standing up to pharmaceutical companies when their products cause harm. We encourage patients to stay informed, maintain open communication with their healthcare providers, and seek legal counsel if they believe they've been adversely affected by a medication.
If you or a loved one has experienced serious side effects while taking a GLP-1 agonist or semaglutide-containing medication, we encourage you to contact us today at (877) 562-0000 or through our online form for a free, no-obligation consultation.